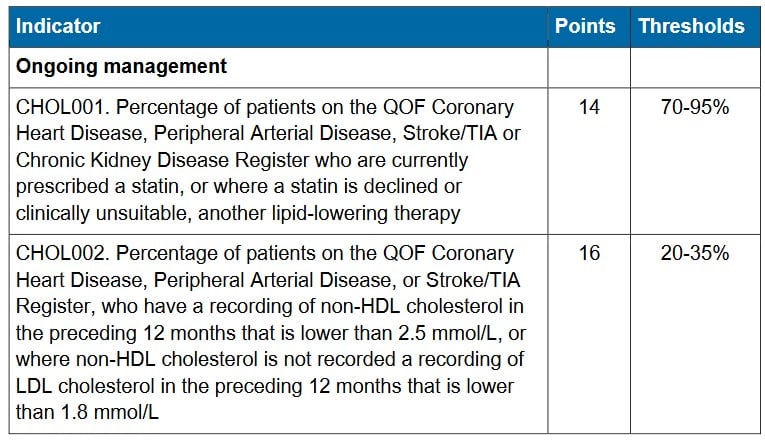
Breaking the Cycle of Recurrent UTIs: An Innovative Non-Antibiotic Approach
Recurrent urinary tract infections (rUTIs) are among the most common and frustrating clinical conditions faced by healthcare professionals. Not only do they impair patients’ quality of life, but they also significantly contribute to antibiotic resistance—a growing global health crisis. A recent groundbreaking study published in Probiotics and Antimicrobial Proteins offers promising evidence that a carefully designed non-antibiotic “bundle” treatment can effectively manage rUTIs, drastically reducing antibiotic use while improving patient outcomes.
Tackling the rUTI Challenge
Women suffering from rUTIs often find themselves in a relentless cycle of infections, symptoms, and antibiotics. The overuse of antibiotics is especially concerning due to the rising prevalence of multidrug-resistant organisms. Recognizing this, researchers from the ASFO Santa Maria degli Angeli Hospital in Pordenone, Italy, investigated a multimodal non-antibiotic strategy, targeting both infection prevention and overall patient wellness.
The Non-Antibiotic Bundle: A Holistic Intervention
The study enrolled 47 women experiencing recurrent UTIs, testing a comprehensive six-month intervention that combined:
- Behavioral Changes: Enhanced hydration (minimum 2 liters daily), improved bowel management, and optimized intimate hygiene practices.
- Phytotherapy: Regular consumption of cranberry extract and D-mannose, both known to inhibit bacterial adhesion to urothelial surfaces.
- Probiotics: A combination of oral and vaginal probiotics containing strains such as Lactobacillus, Bifidobacterium, and Saccharomyces boulardii, aimed at restoring healthy vaginal and intestinal microbiota.
Remarkable Results: Reduced Antibiotic Use and Improved Quality of Life
After six months, the results were impressive:
- 76% reduction in urinary tract infections compared to the previous six months.
- Over 90% decrease in antibiotic exposure, significantly minimizing the risk of antimicrobial resistance.
- Significant improvement in chronic symptoms like abdominal discomfort, urinary urgency, and suprapubic pain.
- Enhanced quality of life reported by over 80% of participants.
Clinical Implications: A Paradigm Shift in UTI Management
The findings suggest that a holistic, multimodal strategy can profoundly change the standard approach to managing rUTIs. By focusing on patient education, lifestyle adjustments, and microbiota health rather than solely relying on antibiotics, clinicians have a powerful new tool to combat recurrent infections.
Moving Forward
Although this initial study had a modest sample size, the outcomes strongly support further large-scale trials. By reducing antibiotic dependence, healthcare providers can not only manage rUTIs more effectively but also contribute to global antibiotic stewardship efforts.
This study represents an important step toward a future where non-antibiotic solutions play a central role in infectious disease management. It’s time to shift our clinical focus towards strategies that not only address symptoms but also promote long-term health and resilience against infections.
Reference:
Venturini, S., Reffo, I., Avolio, M. et al. (2024). The Management of Recurrent Urinary Tract Infection: Non-Antibiotic Bundle Treatment. Probiotics & Antimicro. Prot., 16, 1857–1865. https://doi.org/10.1007/s12602-023-10141-y