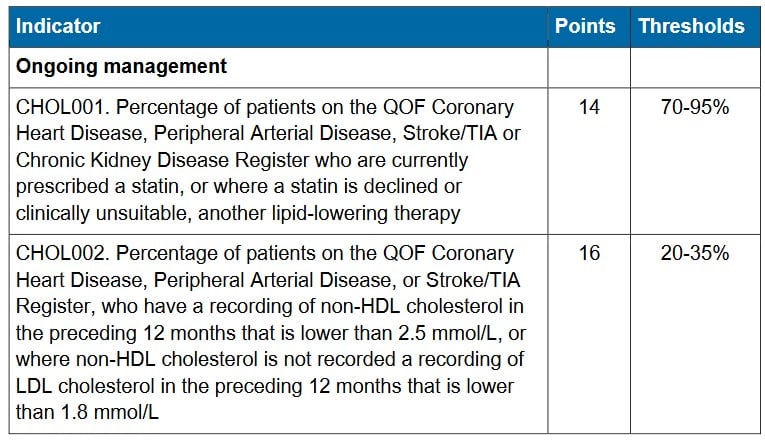
When Benjamin Franklin asked his contacts in France to send over a mysterious elixir for his gout in the 1760s, he probably did not realise he was ordering a medicine that had already been known, mainly as a poison, for more than a millennium. The concoction, sold as Eau Medicinale by French officer Nicolas Husson, crossed the Atlantic as a fashionable gout cure. What Franklin could not have foreseen is that the same substance would one day appear in the New England Journal of Medicine as a tool for preventing myocardial infarction.
The active ingredient in Husson’s remedy was colchicine, derived from the autumn crocus (Colchicum autumnale). Few medicines in routine UK practice can claim a lineage stretching from ancient Greek botanists to modern cardiology guidelines, but colchicine is one of them.
From ‘One-Day Killer’ to Medical Curiosity
The earliest descriptions of colchicum-like plants sit deep in classical literature. Theophrastus, writing in the 4th century BC, described a deadly plant known as ephemeron, literally “the one-day killer” (Theophrastus, Historia Plantarum). By the 1st century AD, Dioscorides had formalised the name kolchikon after the Black Sea region of Colchis (Dioscorides, De Materia Medica). His warnings were unambiguous: used unwisely, colchicum “kills by choking”.
For more than 1,500 years, Western physicians largely followed this advice. Medieval herbals listed colchicum among plants to be admired but not consumed. Renaissance herbalist John Gerard summed it up neatly when he wrote that its roots were “very hurtfull to the stomacke” (Gerard, Herball, 1597).
The 18th Century Breakthrough: Eau Medicinale
Colchicum’s reputation shifted dramatically in the late 1700s when Nicolas Husson marketed Eau Medicinale as a secret gout remedy. It sold at a premium, 22 shillings per tiny bottle, and was widely imitated but poorly understood. European physicians debated its contents, suggesting everything from digitalis to white hellebore.
In 1814, Londoner John Want solved the mystery. Using a mix of literature review, analysis, and possibly a little espionage, Want identified colchicum as the active ingredient. His findings reshaped gout management across Europe almost immediately.
A plant long classified as a poison had become one of medicine’s most reliable abortive treatments for gout flares.
Why Colchicine Works, and Why It Sometimes Does Not
Colchicine binds to tubulin, preventing microtubule formation. This slows or halts cellular processes that depend on structural scaffolding. For neutrophils, this means impaired migration, reduced endothelial adhesion, and blunted inflammatory signalling. Clinically, this results in anti-inflammatory activity at low doses.
Neutrophils conveniently accumulate colchicine because they express low levels of P-glycoprotein. Other tissues pump it out more effectively, which partly explains why the drug can reduce inflammation without universally causing harm.
However, the mechanism also explains the gastrointestinal side effects familiar to clinicians. Blocking microtubules disrupts cell division in rapidly renewing tissues, particularly the gut and bone marrow. Hence, diarrhoea remains as ancient a side effect as the drug itself.
Modern Evidence: From FMF to MI Prevention
Familial Mediterranean Fever
Colchicine’s modern renaissance began in the 1970s when researchers discovered that daily colchicine prevents the attacks of Familial Mediterranean Fever and reduces the risk of secondary amyloidosis (Ozen et al., N Engl J Med, 2016). This marked the transition of colchicine from simple gout treatment to targeted anti-inflammatory therapy.
Acute Gout
Surprisingly, despite centuries of use, high-quality evidence arrived only recently. The AGREE trial (Terkeltaub et al., Arthritis Rheum, 2010) showed that a low-dose regimen (1.8 mg over one hour) was nearly as effective as traditional high-dose therapy while causing fewer adverse effects. This finding rapidly changed practice worldwide.
Pericarditis
Cardiology then embraced colchicine. The ICAP trial (NEJM, 2013) demonstrated that combining colchicine with standard therapy halved recurrence rates in acute pericarditis. This led to its incorporation into ESC guidelines and rapid uptake across Europe.
Coronary Disease
The LoDoCo2 (NEJM, 2020) and COLCOT (NEJM, 2019) trials further broadened interest. Daily low-dose colchicine (0.5 mg) reduced cardiovascular events, including myocardial infarction and stroke, by roughly 23 to 30 percent. These results positioned colchicine as a serious contender in long-term cardiovascular risk reduction.
A once-feared botanical toxin had become a frontline anti-inflammatory drug across multiple specialties.
Regulatory Plot Twist: When Safety Creates a Monopoly
For decades, colchicine in the United States existed in a regulatory grey zone. It was widely manufactured, rarely questioned, and astonishingly cheap.
The FDA’s 2006 Unapproved Drugs Initiative changed that. The programme encouraged companies to fund modern studies in exchange for temporary market exclusivity. URL Pharma undertook the necessary research, including pharmacokinetic studies and the AGREE trial, and gained approval for Colcrys in 2009.
Once approved, the FDA ordered all other unapproved colchicine products off the market. Overnight, a ten-cent pill became a five-dollar pill.
The backlash was swift. US senators accused URL Pharma of exploiting regulation to create a monopoly. Analysts estimated that if all gout patients required daily colchicine at the new price, annual costs could exceed 11 billion dollars.
Generics eventually returned after exclusivity expired, but prices have never returned to the pre-2009 level. The episode became a case study in how well-intended policy can distort markets.
In 2020, partly because of such cases, the programme was formally discontinued.
The Road Ahead
Colchicine’s journey mirrors the evolution of medicine itself. It has travelled from ancient herbal lore to modern pharmacology, from empirical remedies to targeted anti-inflammatory therapy. Its expanding role in cardiovascular medicine continues to attract attention, and further repurposing trials are underway.
Yet the Colcrys episode remains a lasting reminder that the scientific merit of a drug can be overshadowed by regulatory and economic forces. Poorly designed incentives can restrict patient access to medicines that humanity has relied on for centuries.
For UK doctors, colchicine’s story is more than an interesting historical footnote. It is a window into how policy, economics, and pharmacology intersect, and how even the most familiar medicines can have dramatic backstories.
Selected References
-
Theophrastus. Historia Plantarum, 4th century BC
-
Dioscorides. De Materia Medica, 1st century AD
-
Gerard J. The Herball, 1597
-
Terkeltaub R et al. AGREE Trial. Arthritis Rheum. 2010
-
Ozen S et al. FMF Review. N Engl J Med. 2016
-
Imazio M et al. ICAP Trial. N Engl J Med. 2013
-
Nidorf SM et al. LoDoCo2 Trial. N Engl J Med. 2020
-
Tardif J et al. COLCOT Trial. N Engl J Med. 2019
-
FDA Unapproved Drugs Initiative, US HHS, 2006 to 2020